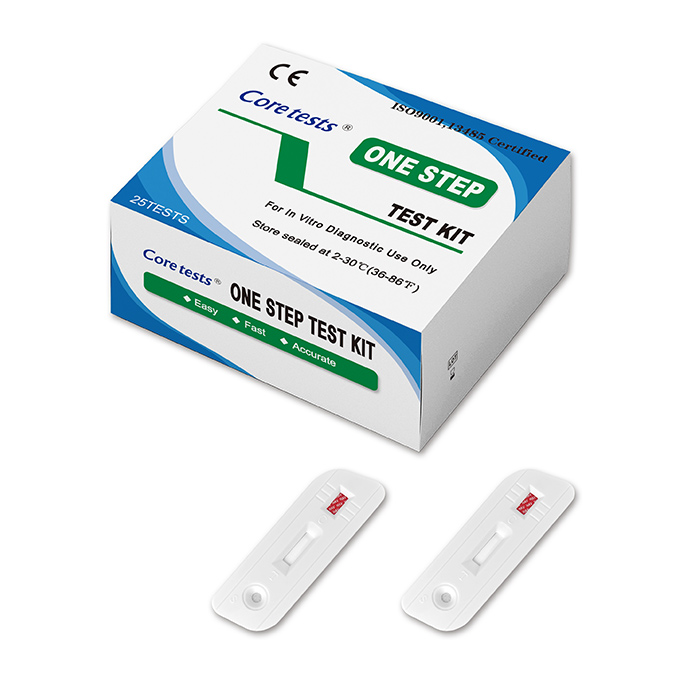
The HAV IgM test cassette is designed as a simple,rapid method for the qualitative determination of lgM response to the hepatitis A virus (HAV) in human blood.This assay is intended for use as a screening test and as an aid in the diagnosis of acute or recent infection (usually 6 months or less) with hepatitis A virus.
TEST PROCEDURE:
- Bring the pouched test cassette to room temperature(15-30℃) prior to testing. Do not open the pouch until ready to perform the assay.
- Remove the test cassette from the sealed pouch. Lay it on a flat, clean and dry surface.
- According to the proportion of 1:1000, add 4μl of specimen in the 4ml buffer bottle and shake well.
- Then drop 2 drops of well mixed buffer into the sample well.
- Interpret test results between 15-20 minutes.Do not interpret the results after 30 minutes.
Positive: Two colored lines are visible in the test result window, one in the test region and another one in the control region, which is used to indicate proper performance of the device. The color intensity of the test line may be weaker or stronger than that of the control line.
Negative: The control line appears in the viewing window, but the test line is not visible.
Invalid: No line appears in the control region. Under no circumstances should a positive sample be identified until the control line forms in the viewing area. If the control line does not form, the test result is inconclusive and the assay should be repeated.
NOTE: Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for the control line failure. Review the procedure and repeat the test with a new cassette. If problem persists, please contact your local distributor.
SPECIFICATION:
| Description | Specimen | Format | Catalog No. | Sensitivity | Package Size | Strip Width | Certificate |
| (HAV)HAV lgM Test | Serum/Plasma | Strip | B01-11 | 96.32% | 25 Tests | 3.0 mm | CE |
| Cassette | B01-20 | 15 Tests | 4.0 mm | ||||
| Whole Blood | B01-21 | 15 Tests | 4.0 mm |

 Fertility Test
Fertility Test Respiratory Infectious Diseases Test
Respiratory Infectious Diseases Test Infectious Diseases Test
Infectious Diseases Test Cardiac Marker Test
Cardiac Marker Test Tumor Marker Test
Tumor Marker Test Health Test
Health Test Blood Glucose Test
Blood Glucose Test CE
CE